Energy levels of an atom's electrons
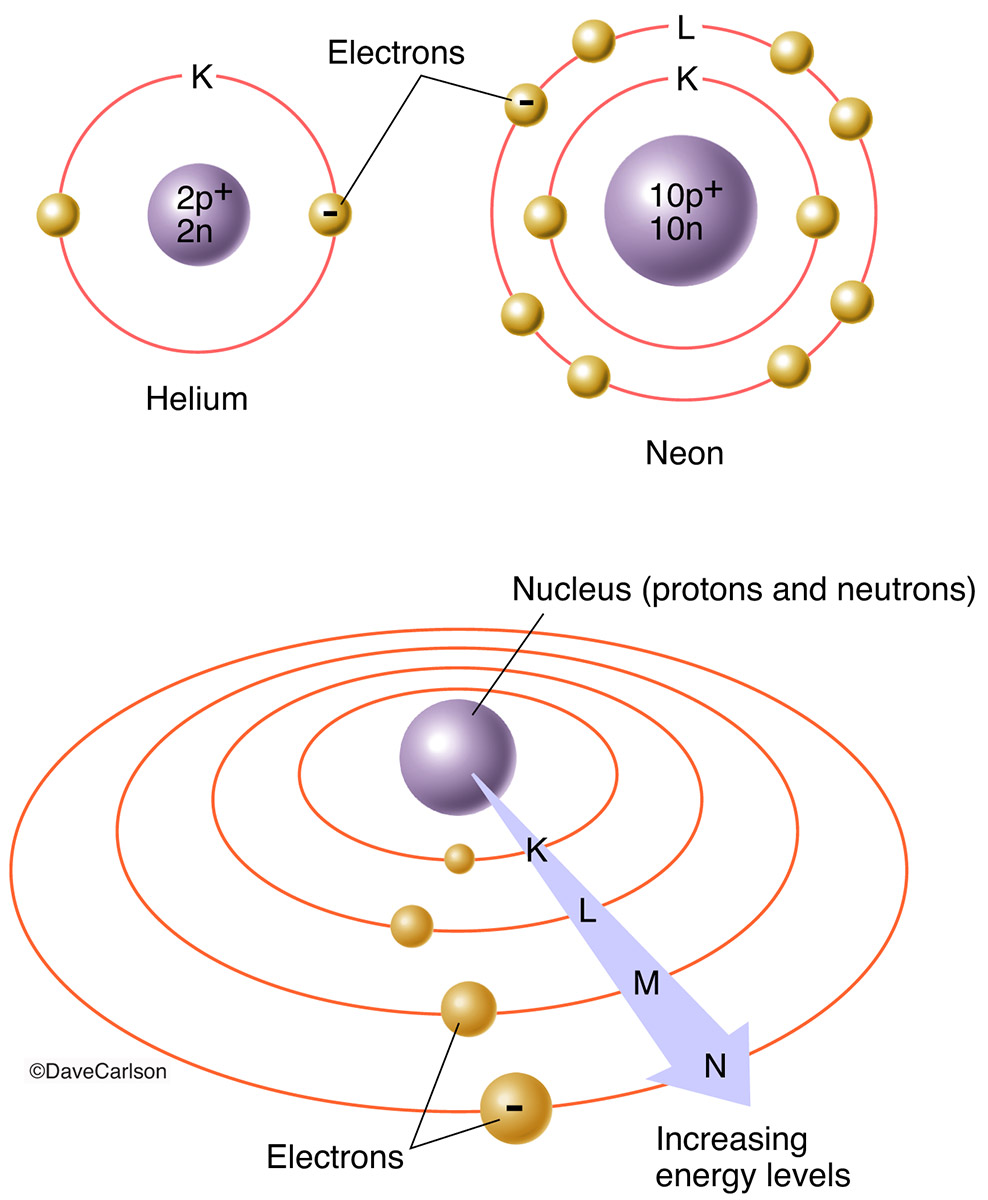
- Campbell figure walkthrough energy levels of an atom's electrons
- First and second energy levels of an atom electrons
- Energy levels of electrons in an atom are quantized
- Energy levels of electrons in an atom are continuous
- The energy levels of an atom are occupied by ____.a.electrons.neutrons.protons.ions
- Describe energy levels of electrons in an atom
- Electrons on the outermost energy levels of an atom are known as
- Energy levels are fixed distances from the ___ of an atom where electrons are found
- The distribution of electrons in energy levels of an atom
- What energy levels would the electrons of an atom with 15 protons occupy
- Energy levels are orbits of electrons surrounding an atom
- The arrangement of electrons in the energy levels of an atom
- Discrete energy levels that contain the electrons of an atom are called
- The distribution of electrons in various energy levels of an atom is called
- How many electrons can the second energy levels of an atom hold