Maximum number of electrons accommodated in l shell
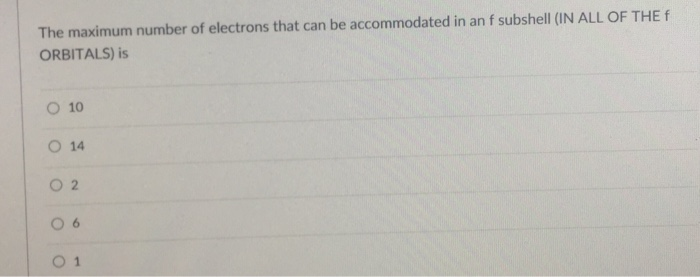
- Maximum number of electrons accommodated in l shell of an atom
- Maximum number of electrons that can be accommodated in l shell
- The maximum number of electrons that can be accommodated in l shell are dash
- The maximum number of electrons that can be accommodated in l shell of an atom will be
- How many maximum number of electrons can be accommodated in l=3 shell
- How many maximum number of electrons can be accommodated in l=4 shell
- How many maximum number of electrons can be accommodated in l 3 sub shell
- How many maximum number of electrons can be accommodated in l 4 sub shell
- The maximum number of electrons that can be accommodated in shell for which l 3 is
- What is the maximum number of electrons that can be accommodated in l m and n shell