N 3 l 2 ml 0
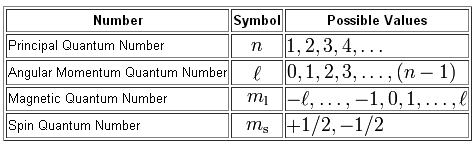
- N 3 l 2 ml 0 how many electrons
- N 3 l 2 ml 0 ms
- N 3 l 2 ml 0 orbital
- N 3 l 2 ml 0 ms 1⁄2
- N 3 l 2 ml 0 ms =- 1 2
- N 3 l 2 ml 0 ms =+ 1 2 name the element
- N 3 l 1 ml 0 ms =- 1 2 element
- The set of quantum numbers n 3 l 2 ml 0
- N 3 l 0 ml 0 ms =+ 1 2 how many electrons
- N 4 l 3 ml 0 ms =+ 1 2
- N 5 l 3 ml 0 ms 1 2
- The maximum number of electrons for n 3 l 2 and ml 0 is
- N 3 l 0 ml 0 ms =+ 1 2 మూలకం పేరు